CAR T cell therapy for the treatment of cancer is one of the breakthrough innovations of modern medicine.
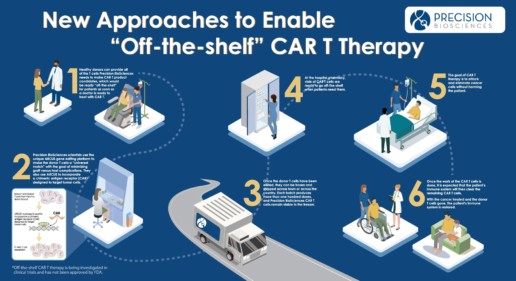
Cancer immunotherapy is a type of cancer treatment that uses the body’s immune system to fight the disease. Currently approved chimeric antigen receptor (CAR) T cell therapies have demonstrated dramatic efficacy in some cancers. However, these patient-derived, autologous CAR T products introduce several important limitations to patient care including patient access, cost, and safety concerns.
What do frozen samurai warriors and magic goggles have to do with treating cancer?
Chief Scientific Officer Derek Jantz explains how ARCUS is used to make allogeneic, donor-derived CAR T cell therapies with the potential to change the way the world battles cancer.
Using ARCUS genome editing, we are advancing a novel pipeline of allogeneic or “off-the-shelf” CAR T cell therapies designed to overcome these known challenges while offering several potential advantages for treating hematologic cancers and solid tumors:
- Healthy donors and healthy T cells
- A single-step editing process
- Optimized T cell characteristics
- Immediate availability

Scientific Publications and Presentations
Allogeneic CAR T cells with Deoxycytidine Kinase Knockdown Demonstrate Resistance to Fludarabine
Pires, M. et al. Journal for ImmunoTherapy of Cancer. 9,6 (2021). Society for Immunotherapy of Cancer (SITC) 2021 Annual Meeting.
Preliminary Safety and Efficacy of PBCAR0191, an Allogeneic, Off-the-Shelf CD19-Targeting CAR-T Product, in Relapsed/Refractory (r/r) CD19+ NHL
Shah, B.D. et al. American Society of Clinical Oncology (ASCO) 2021 Annual Meeting.
Initial Findings of the Phase 1 Trial of PBCAR0191, a CD19 Targeted Allogeneic CAR T Cell Therapy
Jacobson C.A. et al. American Society of Hematology (ASH) 2019 Annual Meeting.
Development of a Clinical-Grade Meganuclease for Allogeneic CAR T Cell Production
Lape, J. et al. American Society of Gene and Cell Therapy (ASGCT) 2018 Annual Meeting.
Off the Shelf T Cell Therapies for Hematologic Malignancies
McCreedy, B. J., Senyukov, V. V., Nguyen, K. (2018) Best Practice & Research Clinical Haematology. 31(2) 166-175. https://doi.org/10.1016/j.beha.2018.03.001
Integration of a CD19 CAR Into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells
MacLeod D.T., Antony J., Martin A.J., (2017) Molecular Therapy. 25(4):949-961. https://doi.org/10.1016/j.ymthe.2017.02.005
Effective Cell Dose and Functional Attributes of Azercabtagene Zapreleucel (Azer-cel; PBCAR0191) Associated with Allogeneic CAR T-Cell Safety and Efficacy in Patients with Relapsed/Refractory B-Cell Lymphoma
Jacobson, C.A., American Society of Hematology (ASH) 2022 Annual Meeting