DURHAM, N.C., Aug. 14, 2019 (GLOBE NEWSWIRE) — Precision BioSciences, Inc. (Nasdaq: DTIL) (“Precision”), a genome editing company dedicated to improving life through the application of its pioneering, proprietary ARCUS® platform, today announced financial results for the second quarter ended June 30, 2019, and provided a corporate update.
Key Highlights and Recent Developments
- Initiated a Phase 1/2a clinical trial of Precision’s first off-the-shelf (allogeneic) chimeric antigen receptor (CAR) T cell therapy candidate, PBCAR0191. PBCAR0191 will be evaluated in adult patients with relapsed or refractory (“R/R”) non-Hodgkin lymphoma (“NHL”) or R/R B-cell precursor acute lymphoblastic leukemia (“B-ALL”).
- Enrolled and dosed patients in the Phase 1/2a clinical trial of PBCAR0191 consistent with expected timelines at three leading US sites with expertise in CAR T cell therapies: Moffitt Cancer Center, Dana Farber Cancer Institute, and City of Hope National Medical Center.
- Enhanced senior leadership team with key appointments including Chief Medical Officer Christopher R. Heery, MD, and General Counsel, Dario Scimeca.
- Presented pre-clinical updates for multiple in vivo gene editing programs at the American Society for Gene and Cell Therapy (ASGCT) Annual Meeting.
- Opened an in-house Current Good Manufacturing Practice (cGMP) compliant manufacturing facility, believed to be the first in the United States dedicated to genome-edited, off-the-shelf CAR T cell therapy product candidates.
- Celebrated Mario Pennisi, Resident Director of wholly-owned subsidiary Elo Life Systems Inc. (Elo Life Systems), being awarded the BIO Leadership and Legacy Award in Industrial Biotech and Agriculture.
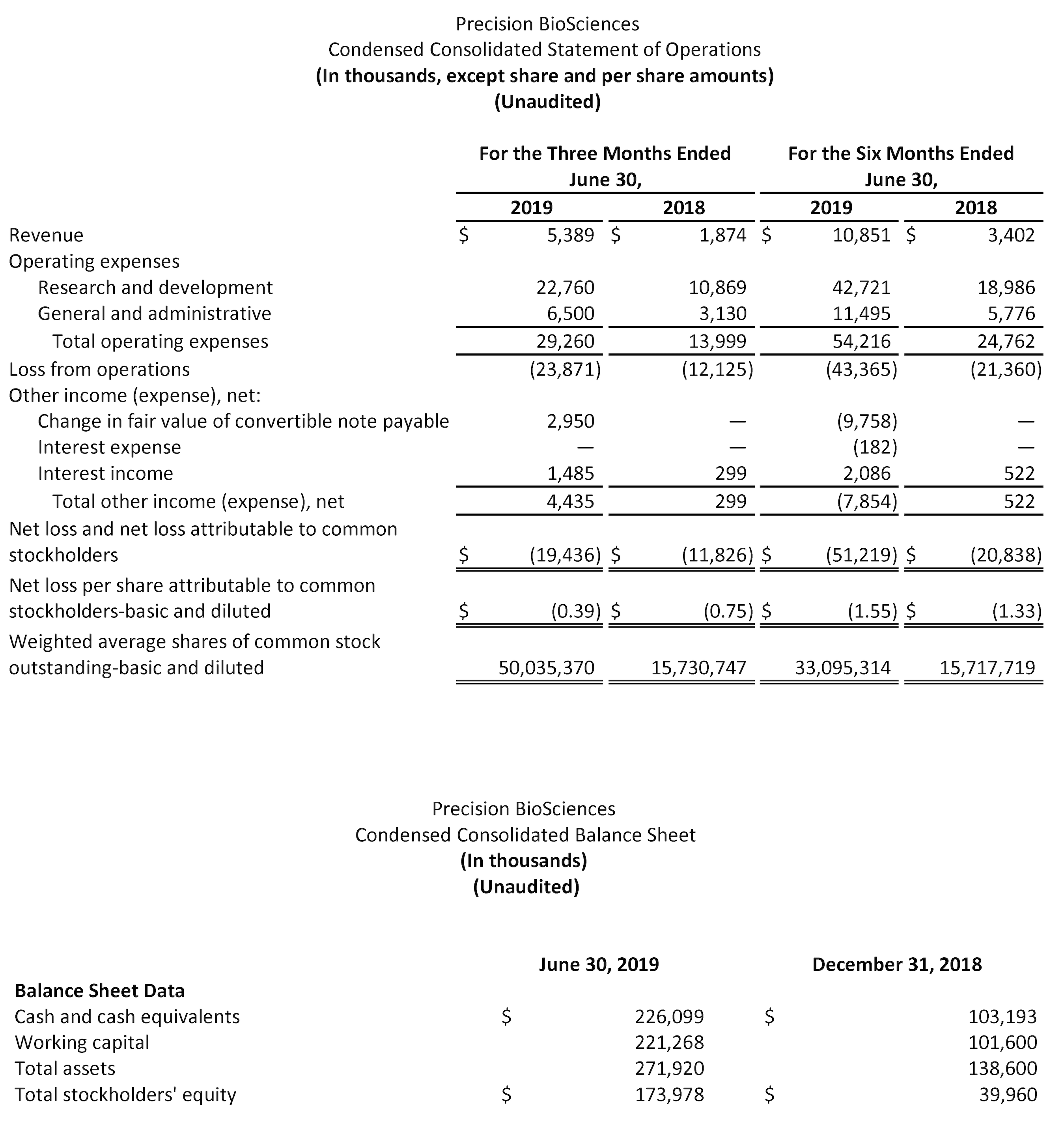
- Ended the quarter with $226 million in cash and cash equivalents, expected to fund operating expenses and capital expenditure requirements into 2021.
“Following the closing of our successful IPO at the beginning of April, we have continued to leverage the unique capabilities of our proprietary ARCUS genome editing platform for therapeutic and human health applications. We achieved a significant milestone in our mission to deliver transformative, off-the-shelf CAR T cell therapies to cancer patients by dosing the first subjects with our lead allogeneic product candidate, PBCAR0191. Our portfolio of in vivo gene correction candidates continues to progress towards the clinic, and we are excited by the ongoing momentum at Elo Life Systems,” commented Matt Kane, Chief Executive Officer and Co-Founder of Precision. “In the second quarter of 2019, we also successfully expanded our senior leadership team and are pleased to welcome Christopher Heery, MD, as Precision’s first Chief Medical Officer, and Dario Scimeca as General Counsel. In addition, we expect that our newly opened, in-house CAR T cell manufacturing facility, which we believe to be the first of its kind in the United States, will be able to support multiple simultaneous clinical trials and, in time, the commercialization of our product candidates, if approved. Our focus on manufacturing continues to demonstrate our commitment to rapidly deliver our advanced therapeutic candidates to patients in need.”
Other Highlights and Upcoming Milestones
Program updates
- On April 15, 2019, Precision initiated dosing of its first allogeneic CAR T cell therapy candidate, PBCAR0191, in a Phase 1/2a clinical trial. PBCAR0191 is being developed in collaboration with Servier, an international pharmaceutical company. Made from donor-derived T cells modified using Precision’s ARCUS genome editing technology, PBCAR0191 recognizes the tumor cell surface protein CD19, an important and validated target in several B-cell cancers, and is designed to avoid graft-versus-host disease, a significant complication associated with donor-derived, cell-based therapies. Precision believes this to be the first US-based clinical trial to evaluate an allogeneic CAR T therapy for R/R NHL. The trial is designed to assess safety and tolerability of PBCAR0191 at increasing dose levels, with secondary objectives including evaluation of anti-tumor activity. Dosing of subjects continues to progress as planned. Precision expects to present interim data from this trial at a scientific conference no later than the first quarter of 2020.
- Precision’s second allogeneic CAR T cell therapy product candidate, PBCAR20A, is expected to enter a Phase 1/2a clinical trial in the fourth quarter of 2019. PBCAR20A is wholly owned by Precision and targets the validated tumor cell surface target CD20. It will be investigated in subjects with two subtypes of NHL, chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
- At the ASGCT Annual Meeting held in Washington, DC from April 29 to May 2, 2019, Precision presented several updates on its in vivo gene editing programs. During the meeting, Precision’s scientists and collaborators shared updates on four pre-clinical programs: hepatitis B (HBV, being developed in partnership with Gilead), autosomal dominant retinitis pigmentosa (adRP), primary hyperoxaluria (PH1), and familial amyloid polyneuropathy (FAP). Discussions with the US Food and Drug Administration (FDA) for the HBV program are planned for the second half of 2019.
Senior management additions
- On May 14, 2019, Precision announced the first of two significant hires in the second quarter with Christopher R. Heery, MD, joining as the Company’s first Chief Medical Officer (CMO). Dr. Heery has strong experience managing translational clinical programs, having served as head of the Clinical Trials Group of the Laboratory of Tumor Immunology and Biology at the National Cancer Institute where he was the lead associate investigator of the Phase 1 study of the anti-PD1 immunotherapy avelumab. He also served as CMO at Bavarian Nordic and is board certified in both medical oncology and internal medicine.
- On June 12, 2019, Precision announced the appointment of Dario Scimeca as General Counsel. Mr. Scimeca joined Precision from Genentech where he supported the development and commercialization of multiple drug products including Rituxan®, Tecentriq®, Gazyva®, Venclexta® and Hemlibra® as associate general counsel. A graduate of UC Berkeley Law School, in his previous roles Mr. Scimeca has overseen US FDA and European Medicines Agency regulatory matters, managed securities and intellectual property litigation, and been responsible for commercial contracts and compliance matters.
Manufacturing Center for Advanced Therapeutics
- On July 18, 2019, Precision announced the opening of its in-house cGMP compliant manufacturing facility, located in Research Triangle Park, North Carolina, which the Company believes to be the first in the United States dedicated to genome-edited, off-the-shelf CAR T cell therapy product candidates. The Manufacturing Center for Advanced Therapeutics (MCAT) is designed to enable in-house production of three different drug substances: allogeneic CAR T cells, messenger RNA (including formulations development) and adeno-associated viral vectors. Precision intends to use this new manufacturing center to create clinical trial material for its planned Phase 1/2 clinical trials starting in 2020. In the longer term, Precision believes MCAT has the potential to be a commercial launch facility with the capacity to generate up to 10,000 allogeneic doses of CAR T cell therapies and 4,000 doses of gene therapies per year.
Elo Life Systems
- Also in July 2019, the Company’s wholly owned subsidiary, Elo Life Systems, which is dedicated to creating novel products that enhance the nutrition and diversity of the global food supply, announced that Mario Pennisi, Resident Director of Elo Life Systems – Australia was awarded the prestigious BIO Leadership and Legacy Award in Industrial Biotech and Agriculture. The award is presented to individuals who have shown exemplary leadership and dedicated a significant portion of their career to advancing industrial biotechnology and growing the bio-based economy. This award further demonstrates the depth of talent at Precision, and the pioneering work ongoing at Elo Life Systems.
Upcoming Corporate Presentations
Precision’s senior management team will be presenting and meeting with investors at the following upcoming conferences:
- Baird Global Healthcare Conference, New York, NY, Sept. 4 – 5, 2019
- Presentation and panel discussion at The Alliance for Regenerative Medicine’s Cell and Gene Meeting on the Mesa, Carlsbad, CA, Oct. 2 – 4, 2019
- Chardan Genetic Medicines Conference, New York, NY, Oct. 7 – 8, 2019
- Jefferies Gene Therapy Summit Presentation, New York, NY, Oct. 9, 2019
- Stifel 2019 Healthcare Conference, New York, NY, Nov. 19 – 20, 2019
- Jefferies London Healthcare Conference, London, UK, Nov. 20 – 21, 2019
- Piper Jaffray Annual Health Care Conference, New York, NY, Dec. 3 – 5, 2019
Second Quarter 2019 Financial Results
Cash and Cash Equivalents: As of June 30, 2019, Precision had approximately $226.1 million in cash and cash equivalents. We expect that existing cash and cash equivalents will be sufficient to fund operating expenses and capital expenditure requirements into 2021.
Revenues: Total revenues for the quarter ended June 30, 2019 were $5.4 million, compared to $1.9 million for the quarter ended June 30, 2018. This increase was primarily due to research funding from our joint development collaboration partners.
Research and Development Expenses: Research and development expenses were $22.8 million for the quarter ended June 30, 2019, as compared to $10.9 million for the same period in 2018. This increase of $11.9 million was primarily due to increases in platform development and early-stage research expenses due to contract manufacturing costs and an increase in personnel costs and expenses to support our technology platform development and manufacturing capabilities, partially offset by a decrease in direct research and development expenses related to our CD19 program.
General and Administrative Expenses: General and administrative expenses were $6.5 million for the quarter ended June 30, 2019, as compared to $3.1 million for the same period in 2018. The increase of $3.4 million was primarily due to employee-related costs for our additional personnel and facility costs associated with our growing infrastructure needs.
Net Loss: Net loss was $19.4 million, or $(0.39) per share, for the quarter ended June 30, 2019, compared to a net loss of $11.8 million, or $(0.75) per share, for the same period in 2018.
About Precision BioSciences, Inc.
Precision BioSciences is dedicated to improving life (DTIL) through its proprietary genome editing platform, “ARCUS.” Precision leverages ARCUS in the development of its product candidates, which are designed to treat human diseases and create healthy and sustainable food and agriculture solutions. Precision is actively developing product candidates in three innovative areas: allogeneic CAR T immunotherapy, in vivo gene correction, and food. For more information regarding Precision, please visit www.precisionbiosciences.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding the promise and potential impact of our ARCUS genome editing technology, expectations for our in vivo gene therapy candidates, timing of our discussions with the FDA, the performance of Elo Life Systems, the timing, objectives, status and the results of clinical studies of our CAR T product candidates and the capabilities of our manufacturing facility. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “could,” “expect,” “should,” “plan,” “intend,” “estimate,” “target,” “mission,” “may,” “will,” “would,” “should,” “could,” “target,” “project,” “predict,” “contemplate,” “potential,” or the negative thereof and similar words and expressions.
Forward-looking statements are based on management’s current expectations, beliefs and assumptions and on information currently available to us. Such statements are subject to a number of known and unknown risks, uncertainties and assumptions, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various important factors, including, but not limited to, our ability to become profitable; our ability to procure sufficient funding and requirements under our current debt instruments; our limited operating history; our ability to identify, develop and commercialize our product candidates; our dependence on our ARCUS technology; the initiation, cost, timing, progress and results of research and development activities, preclinical or greenhouse studies and clinical or field trials; our or our collaborators’ ability to identify, develop and commercialize product candidates; our or our collaborators’ ability to advance product candidates into, and successfully complete, clinical or field trials; our or our collaborators’ ability to obtain and maintain regulatory approval of future product candidates, and any related restrictions, limitations and/or warnings in the label of an approved product candidate; the regulatory landscape that will apply to our and our collaborators’ development of product candidates; our ability to achieve our anticipated operating efficiencies as we commence manufacturing operations at our new facility; our ability to obtain and maintain intellectual property protection for our technology and any of our product candidates; the potential for off-target editing or other adverse events, undesirable side effects or unexpected characteristics associated with any of our product candidates; the success of our existing collaboration agreements; our ability to enter into new collaboration arrangements; public perception about genome editing technology and its applications; competition in the genome editing, biopharmaceutical, biotechnology and agricultural biotechnology fields; potential manufacturing problems associated with any of our product candidates; potential liability lawsuits and penalties related to our technology, our product candidates; and our current and future relationships with third parties; and other important factors discussed under the caption “Risk Factors” our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2019, as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov.
All forward-looking statements speak only as of the date of this press release and, except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Investor Contact:
Jason Wong
Blueprint Life Science Group
Tel. (415) 375-3340 Ext. 4
jwong@bplifescience.com
Media Contact:
Cory Tromblee
Scient Public Relations
Tel. (617) 571-7220
cory@scientpr.com