ARCUS is a proprietary genome editing technology discovered by scientists at Precision BioSciences
ARCUS is based on a naturally occurring genome editing enzyme, I-CreI, that evolved in the algae Chlamydomonas reinhardtii to make highly specific cuts and DNA insertions in cellular DNA. I-CreI is a member of a larger class of enzymes called homing endonucleases or meganucleases.
The unique advantages of ARCUS relate to type of cut, small size, and simplicity

- 3 Prime Overhang Cut
- Homology-Directed Repair (HDR)
- Complementary overhangs drive “Perfect” Re-ligation

- Smallest gene editor (~1500 bp)
- Enables delivery of MORE payload, which then enables sophisticated edits
- Delivery, both non-viral and viral, to diverse tissues in the body

- Only single component editor that recognizes and cuts DNA
- Single component streamlines delivery and results in the highest efficiency
- Single component editor requires lower dose of delivery vehicle
ARCUS for the More Sophisticated Gene Edit
Designed by nature for a multitude of applications versus other gene editing modalities
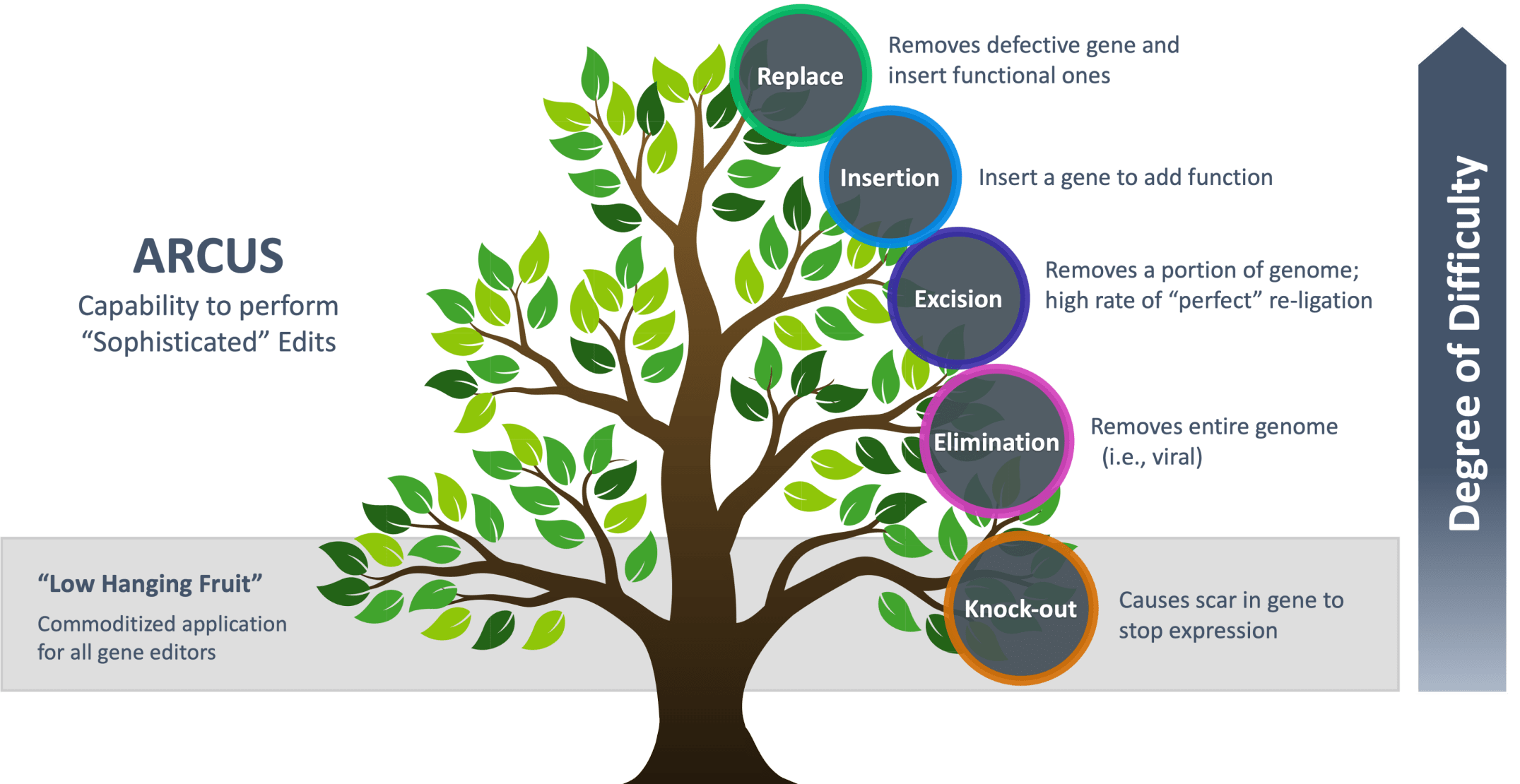
Wholly owned programs include:
PBGENE-HBV
PBGENE-HBV is being developed for the treatment of patients with chronic hepatitis B, with submission of a clinical trial application (CTA) and/or investigational new drug (IND) application targeted for 2024. During the R&D Day, Precision showed data highlighting the potential of PBGENE-HBV to eliminate covalently closed circular DNA and inactivate integrated hepatitis B virus (HBV) DNA to drive durable antigen loss, leading to a potential functional cure.
PBGENE-DMD (Muscle Targeted Excision Program)
PBGENE-DMD is Precision’s development program for the treatment of DMD. DMD is a genetic disease caused by mutations in the dystrophin gene that prevent production of the dystrophin protein and affects approximately 15,000 patients in the U.S. alone. There are currently no approved therapies that can drive durable and significant functional improvements. PBGENE-DMD is designed to improve function over time and addresses more than 60% of patients with DMD by employing two complementary ARCUS nucleases delivered in a single AAV to excise exons 45-55 of the dystrophin gene with the aim of restoring the body’s native production of a functional dystrophin protein that more closely resembles normal dystrophin than the truncated microdystrophins being marketed and developed in the clinic.
PBGENE-3243
PBGENE-DMD is Precision’s development program for the treatment of DMD. DMD is a genetic disease caused by mutations in the dystrophin gene that prevent production of the dystrophin protein and aƯects approximately
15,000 patients in the U.S. alone. There are currently no approved therapies that can drive durable and significant functional improvements. PBGENE-DMD is designed to improve function over time and addresses more than 60% of
patients with DMD by employing two complementary ARCUS nucleases delivered in a single AAV to excise exons 45-55 of the dystrophin gene with the aim of restoring the body’s native production of a functional dystrophin protein
that more closely resembles normal dystrophin than the truncated microdystrophins being marketed and developed in the clinic.

